|
|
||||||||||
|
Coxiella burnetii. Coxiella burnetii антибиотикиCoxiella burnetii - microbewikiA Microbial Biorealm page on the genus Coxiella burnetii ClassificationHigher order taxaBacteria; Proteobacteria; Gammaproteobacteria; Legionellales; Coxiellaceae; Coxiella (1)Bacteria; Proteobacteria; Gammaproteobacteria; Legionellales; Coxiellaceae; Coxiella (1) SpeciesCoxiella burnetii Description and significanceCoxiella burnetii is an obligate intracellular Gram-negative coccobacillus bacterium that is known to be the main pathogen that causes Q fever in mammals and humans. (3) Harold Cox and MacFarlane Burnet initially identified Q fever as “query fever” in 1935 when a number of infections were found to be from an Australian slaughterhouse. Once this disease was elucidated to be a human pathogen, the name was properly changed to Q fever. (4) Its global pathogenic effect demonstrates the need for preventive measures to control the rate of infection worldwide and its potential use for bioterrorism. The significance of a completed sequenced genome is the benefit of being able to further understand the mechanisms of pathogenesis and use this knowledge to fight against this infectious disease. Sheep, cattle, and goats are major sources of Coxiella burnetii that can potentially help spread the disease to other organisms. The most common mode of transmission to humans is primarily through external waste excretions from infected animals. The aerosol route to infection is frequent by inhalation of contaminated air that contains many of these organisms or through an insect vector. (5) Coxiella burnetii live in a wildlife livestock environment and can withstand heat, dryness, and antibacterial compounds, allowing this bacterium to persist outside the host for an extensively long period of time. It is an acidophile, meaning it tends to surround itself in an environment with low pH. Uniquely enough, it can be endocytosed by a macrophage and complete replication inside the phagolysozyme during its life cycle. (3) Genome structureRandom shotgun method was used to sequence the genome of Coxiella burnetii. (3) The genome of this organism contains a single 1,995,281 base-pair circular chromosome and a single 37,393 base-pair Qph2 circular plasmid. (1) In the chromosome, there are 1,022 protein-coding genes found for known proteins, 179 genes for proteins of unknown function, 3 stable rRNAs, and 42 stable tRNAs. Percent of G+C content is approximately 42.6% and percent coding is approximately 89.1% in the chromosome. As for Qph2, there are 11 genes found for known proteins, 5 for proteins of unknown function, and no stable RNAs. 39.3% represents the percent of G+C content and 78.8% is the percent coding in the plasmid. (6) Examining 20 highly conserved proteins in 16S rRNA gene sequence analysis proved the fundamental phylogenetic difference with the α-proteobacterial Rickettsia organisms and confirmed that Coxiella are truly γ-proteobacterial. In genomic comparison with other similar obligate parasites such as those from Rickettsia, the genome of Coxiella was found to contain mobile elements, metabolic and transport capabilities not typically found for the kind of bacteria they are. In addition, 29 insertion sequences were located in the genome; this is noteworthy information because other obligate parasites have very little or none of these elements. However, the presence of pathogenecity islands along with the unique transposons found in the genome does not suggest recent exchange of mobile genetic elements with other organisms. A notion that Coxiella burnetii are enduring a genome reduction, in which certain genes that encode important genetic information ultimately lose their functionality and degrade, has been proposed due to the fact that 83 pseudogenes have been identified. Proteins synthesized by this bacterium has a particularly high pI, which may be due to the acidic environments Coxiella burnetii reside in. (3) Cell structure and metabolismBeing that Coxiella burnetii is a Gram-negative bacterium, this distinction marks important properties about the cell structure. Gram-negative bacteria have two membranes, an inner and outer membrane. The outer membrane lacks an energy source, but compensates by having porins fused into the membrane. The organism does not have a thick cell wall composed of peptidoglycan like Gram-positive bacteria. In between the two membranes lies the periplasmic space. Lipopolysaccharides are anchored to the membrane. During its life cycle, phagocytosis brings the bacteria into the host cell, where it remains in phagocytic vacuoles and replicates in the phagolysozyme. Compared to free-living bacteria, Coxiella burnetii does not have a broader range of transport capacities. In spite of this limitation, this bacterium possesses more transport capacities than other obligate parasites like Rickettsia organisms. For active transport mechanisms by ion-coupled transport, Na+/H+ exchangers are thought to be significant in maintaining pH in the acidic environment Coxiella burnetii thrive in. Although group transfer mechanisms via the phosphotransferase system are not evident in Coxiella, enzymes involved in the system, such as HPr and enzyme 1, are present and play a regulatory role. (3) Unlike the other obligate parasites, the greater metabolic capacities of Coxiella burnetii permit the bacteria to undergo glycolysis, gluconeogenesis, pentose phosphate pathway, and TCA cycle. They lack ATP/ADP exchangers in their transport system. The bacterium utilizes few sugars, including xylose and glucose, and their uptake is aided by a membrane gradient. Amino acids are taken into the cell by an outstanding number of 15 transporters and peptides have three. Due to the copious number of transporters required for the uptake of these organic nutrients, amino acids and peptides may be the major carbon sources for this bacterium. (3) EcologyCoxiella burnetii does not display a pathogenic effect for all organisms it encounters. For example, B. La Scola and D. Raoult discovered that Coxiella burnetii and free-living amoeba Acanthamoeba castellanii are able to co-exist in a benign relationship upon infection. The bacterium was able to produce spore-like structures in vacuoles within the amoeba, demonstrating its ability to differentiate without any interference from the other species. The bacterium does not cause any diseases in the amoeba. Likewise, the amoeba did not utilize the bacterium as food, so it contributes to the survival of Coxiella burnetii by supplying an intracellular niche for it to differentiate into a structure similar to a spore and resist harmful environmental conditions. (7) Besides surviving in a host cell, Coxiella burnetii can adapt to a shared intracellular niche with another parasite without inhibition of development for either. According to research accomplished by Andreoli and colleagues, Vero cells that were previously inhabited by Coxiella burnetii were co-infected with another type of parasite such as Trypanosoma cruzi. Parasitophorous vacuoles (PV) of T. cruzi trypomastigotes and bacterial vacuoles fused together upon co-infection. T. cruzi was able to proceed with differentiation into amastigotes and division in Coxiella burnetii vacuoles. The co-existence of both parasites depends on the ability of Coxiella burnetii to provide sufficient amount of nutrients for T. cruzi. (8) PathologyQ fever is a global disease caused by the pathogen Coxiella burnetii. Without any symptoms and a low dosage that leads to infection, the disease can go unnoticed until serious health consequences begin to present themselves. Because of its natural high resistance to harsh environmental conditions, including dessication, heat, and antibacterial compounds, the transmission of Q fever to other organisms is very effective through contaminated air, the main mode of transmission. In reference to virulence factors, genes encoding adhesive structures in the genome such as pili are absent, but there are 13 ankyrin domains that may assist in the bacterium’s attachment to its host. (3) The method in which humans get infected is by infected animals such as sheep, cattle, goat, dogs, and cats. These infected animals can produce excretions through urine, feces, and milk that contain infectious dosages of this pathogenic bacterium, which can be dangerously mistakenly inhaled, consumed, or be in contact with. The bacterium can be isolated in the placentas of infected animals and can cause abortions due to inflammation. Not just livestock and domestic animals can get infected. Even fish and rodents can acquire Q fever as well. (9) Humans who are exposed to or handle infected animals, such as farmers or veterinarians, have a higher risk of infection and then the disease develops. Typically, there are no obvious symptoms after infection. (9) Only about 50% of people who are infected show signs of the disease. Symptoms similar to the flu may appear, but it is not specific and not always diagnosed as Q fever. The disease takes the form of pneumonia or hepatitis commonly. As the infection becomes more serious, chronic Q fever develops. Endocarditis, inflammation of the aortic heart valves, has been associated with the chronic complications of the disease. The survival rate is higher for those who do not suffer from chronic Q fever. (5) Application to BiotechnologyCoxiella burnetii can have a variety of biological uses in other organisms by producing many multiple clones of its own genes and inserting a gene of interest into the DNA of other organisms that may not have been able to express a certain function before. For example, the genome of Coxiella burnetii contains a gene mucZ that encodes for the production of capsule in E. coli. The gene mucZ can be cloned into a plasmid vector in the host E. coli. The plasmid DNA can be extracted and transferred to E. coli to express mucZ and allow it to produce a capsule for different functions such as motility, attachment, virulence, protection etc. Through PCR, DNA replication can be amplified and useful for studying gene expression or regulation in other organisms with the assistance of plasmids. (10) Current ResearchIn April 2007, scientific research was accomplished by Jensen T.K. and colleagues regarding the presence of Coxiella burnetii in 90 aborted placentas of mammals over a two year period. Fluorescent in situ hybridization (FISH) assay can be implemented as a diagnostic tool to study and differentiate various microbes. They used this method to target 16S ribosomal RNA to identify Coxiella burnetii in formalin-fixed, paraffin-embedded tissue samples. Positive controls were taken from human serum for 12 cases of placentitis and 7 samples for negative controls and compared to the tissue samples. Each sample was hybridized with a specific oligonucleotide probe for the bacterium. All cases yielded a positive result for the bacterium, in which an enlarged trophoblast cytoplasm can be seen due to the presence of reddish brown stained bacteria. It was concluded that FISH was an effective tool in the detection of Coxiella burnetii. (11) Scientific research performed by Kelly Cairns et al. in November 2006 investigated the prevalence of Coxiella burnetii DNA in uterine and vaginal samples from 50 shelter and 47 client-owned healthy female cats of north Colorado with polymerase chain reaction (PCR) assay. The life span of most cats did not reach more than three years. Transmission of Q fever from domestic animals to humans has been reported in past cases, supporting the perception that cats harbor a good source of this bacterium. Antibodies against this bacterium have been found in cats from different countries, including North America, Japan, and Korea. The abundance of antibodies worldwide suggests a global threat to humans who are in contact with cats. Positive controls for the presence of Coxiella burnetii originated from immunofluorescence assay slides. The difficulty in studying this organism in culture points to the use of PCR to track infection in domestic animals and humans. The PCR assay utilized primers specific for Trans1 and Trans2 transposon-like region. The uterine samples that tested positive for Coxiella burnetii DNA came from cats that had a home and owners to take care of them. Thus, it has been shown that healthy cats can harbor this pathogenic bacterium even if they look “clinically normal.” It is stated that parturient cats are more likely to be associated with the transmission of Q fever through cat contact. (12) Not only are methods of isolation and detection of bacteria popular in current microbiology research, the significance of regulation of food safety has not gone unstudied. Because Coxiella burnetii has been known to possibly be found in milk, milk pasteurization has never been so crucial in the promotion of sanitary food production. Milk pasteurization aims to kill all harmful bacteria through extensive heat treatment. Due to the fact that this bacterium is perceived to be one of the toughest heat resistant organisms existing, questions were raised from O. Cerf and R. Condron involving the effectiveness of upholding the requirement for milk to be pasteurized under rigorous temperature and time-sensitive conditions because of previous notions that Q fever could possibly be transmitted through food. The conclusion was that infection arising from the consumption of unpasteurized milk does not inevitably lead to the clinical disease of Q fever, brought on by either inhalation or arthropod bites. (13) References1. ExPASy. Swiss Institute of Bioinformatics. 02 May 2007 <http://expasy.org/sprot/hamap/COXBU.html>. 2. NIAID Biodefense Image Library. National Institute of Allergy and Infectious Diseases. 02 May 2007 <http://www3.niaid.nih.gov/biodefense/Public/Images.htm>. 3. Seshadri R, Paulsen IT, Eisen JA, et al. “Complete genome sequence of the Q-fever pathogen coxiellaburnetii”. PNAS. 2003. Volume 100. p. 5455-5460. 4. Q-fever (Coxiella burnetii) Zoonoses. Animal Disease Diagnostic Laboratory. 02 May 2007 < http://www.addl.purdue.edu/newsletters/2004/spring/qfever.htm>. 5. Q fever. Centers for Disease Control and Prevention. 02 May 2007 <http://0-www.cdc.gov.mill1.sjlibrary.org/ncidod/dvrd/qfever/>. 6. Genome Project. <http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?CMD=Add%20to%20Clipboard&DB=genomeprj>. 7. B. La Scola, D. Raoult. “Survival of Coxiella burnetii within free-living amoeba Acanthamoeba castellanii”. Clinical Microbiology and Infection. 2001. Volume 7 (2). p. 75–79. 8. Andreoli W.K., Taniwaki N.N., Mortara R.A. “Survival of Trypanosoma cruzi metacyclic trypomastigotes within Coxiella burnetii vacuoles: Differentiation and replication within an acidic milieu”. Microbes and Infection. 2006. Volume 8 (1). p. 172-182. 9. Marrie, J. Thomas. “Q fever - a review”. Canadian Veterinary Journal. 1990. Volume 31 (8). p. 555-563. 10. M Zuber, T A Hoover, and D L Court. “Analysis of a Coxiella burnetii gene product that activates capsule synthesis in Escherichia coli: requirement for the heat shock chaperone DnaK and the two-component regulator RcsC”. Journal of Bacteriology. 1995. Volume 177(15). p. 4238–4244. 11. Jensen TK, Montgomery DL, Jaeger PT, Lindhardt T, Agerholm JS, Bille-Hansen V, Boye M. “Application of fluorescent in situ hybridisation and immunohistochemistry for demonstration of Coxiella burnetii in placentas from ruminant abortions”. APMIS. 2007. Volume 115. p. 347–53. 12. Kelly Cairns, Melissa Brewer and Michael R. Lappin. “Prevalence of Coxiella burnetii DNA in vaginal and uterine samples from healthy cats of north-central Colorado”. Journal of Feline Medicine & Surgery. 2007. Volume 9. p. 196-201. 13. O. Cerf and R. Condron. “Coxiella burnetii and milk pasteurization: an early application of the precautionary principle?” Epidemiology and Infection. 2006. p. 946-951. Edited by Lisa Leung, student of Rachel Larsen and Kit Pogliano microbewiki.kenyon.edu Coxiella burnetii - WikipediaCoxiella burnetii is an obligate intracellular bacterial pathogen, and is the causative agent of Q fever. The genus Coxiella is morphologically similar to Rickettsia, but with a variety of genetic and physiological differences. C. burnetii is a small Gram-negative bacterium that is highly resistant to environmental stresses such as high temperature, osmotic pressure, and ultraviolet light. These characteristics are attributed to a small cell variant form of the organism that is part of a biphasic developmental cycle, including a more metabolically and replicatively active large cell variant form.[1] It can survive standard disinfectants, and is resistant to many other environmental changes like those presented in the phagolysosome.[2] History and naming[edit]Research in the 1920s and 1930s identified what appeared to be a new type of Rickettsia, isolated from ticks, that was able to pass through filters. The first description of what may have been Coxiella burnetii was published in 1925 by Hideyo Noguchi, but since his samples did not survive, it remains unclear as to whether it was the same organism. The definitive descriptions were published in the late 1930s as part of research into the cause of Q fever, by Edward Holbrook Derrick and Macfarlane Burnet in Australia, and Herald Rea Cox and Gordon Davis at the Rocky Mountain Laboratory (RML) in the United States.[3] The RML team proposed the name Rickettsia diaporica, derived from the Greek word for having the ability to pass through filter pores, to avoid naming it after either Cox or Davis if indeed Noguchi's description had priority. Around the same time, Derrick proposed the name Rickettsia burnetii, in recognition of Burnet's contribution in identifying the organism as a Rickettsia. As it became clear that the species differed significantly from other Rickettsia, it was first elevated to a subgenus named after Cox, Coxiella, and then in 1948 to its own genus of that name, proposed by Cornelius B. Philip, another RML researcher.[3] Coxiella was difficult to study because it could not be reproduced outside a host. However, in 2009, scientists reported a technique allowing the bacteria to grow in an axenic culture and suggested the technique may be useful for study of other pathogens.[4] Pathogenesis[edit]Immunohistochemical detection of C. burnetii in resected cardiac valve of a 60-year-old man with Q fever endocarditis, Cayenne, French Guiana, monoclonal antibody against C. burnetii and hematoxylin were used for staining: Original magnification ×50The ID50 (the dose needed to infect 50% of experimental subjects) is one via inhalation; i.e., inhalation of one organism will yield disease in 50% of the population. This is an extremely low infectious dose (only 1-10 organisms required), making C. burnetii one of the most infectious known organisms.[5][6] Disease occurs in two stages: an acute stage that presents with headaches, chills, and respiratory symptoms, and an insidious chronic stage. While most infections clear up spontaneously, treatment with tetracycline or doxycycline appears to reduce the symptomatic duration and reduce the likelihood of chronic infection. A combination of erythromycin and rifampin is highly effective in curing the disease, and vaccination with Q-VAX vaccine (CSL) is effective for prevention of it.[citation needed] The bacteria use a type IVB secretion system known as Icm/Dot (intracellular multiplication / defect in organelle trafficking genes) to inject effector proteins called Ank proteins into the host. These effectors increase the bacteria's ability to survive inside the host cell. In Legionella pneumophila, which uses the same secretion system and also injects Ank proteins, survival is enhanced because these Ank proteins interfere with fusion of the bacteria-containing vacuole with the host's degradation endosomes.[7] Use as a biological weapon[edit]The United States ended its biological warfare program in 1969. When it did, C. burnetii was one of seven agents it had standardized as biological weapons.[8] Genomics[edit]At least five completely sequenced genomes of Coxiella burnetti exist,[9] which contain about 2.1 Mbp of DNA each and encode around 2,100 open reading frames; 746 (or about 35%) of these genes have no known function. In bacteria small regulatory RNAs are activated during stress and virulence conditions. Coxiella burnetii small RNAs (CbSRs 1, 11, 12, and 14) are encoded within intergenic region (IGR). CbSRs 2, 3, 4 and 9 are located antisense to identified ORFs. The CbSRs are up-regulated during intracellular growth in host cells.[10] Additional images[edit]
References[edit]
External links[edit]en.wikipedia.org Coxiella - microbewikiA Microbial Biorealm page on the genus Coxiella 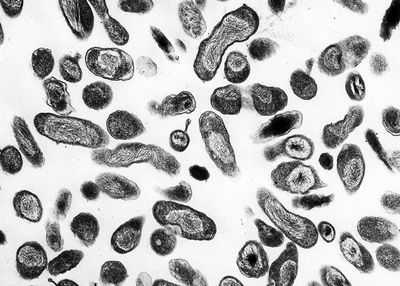 ClassificationHigher order taxa:Bacteria; Proteobacteria; Gammaproteobacteria; Legionellales; Coxiellaceae Species:Coxiella burnetii, Coxiella symbiont of Carios capensis Description and SignificanceCoxiella is a genus best known for the species Coxiella burnetii, the causative agent of Q fever. Q fever affects numerous mammals, although not in the same way. In humans, this disease has a wide range of severity, depending on the initial health of the host, as well as other factors. Instances in Q fever have dropped dramatically in the United States and other developed nations. This is because animals such as cows and goats harbor Coxiella burnetii in large amounts, providing many opportunities for those working in agriculture to become infected. As the United States and other countries have relied more on industrial technology instead of agrarian technology, chances for exposure have decreased, causing the drop in number of infections. Genome StructureThe C. plasmid Qph2 was first sequenced in 1993. Work on the genome structure of Coxiella is still in progress. Another plasmid, Coxiella burnetii plasmid QpDV, was sequenced in 1999. Work has also been done on Coxiella burnetii RSA 493. One of the more recent developments is the sequencing of Coxiella burnetii RSA 493 plasmid pQph2, completed in 2003. Cell Structure and Metabolism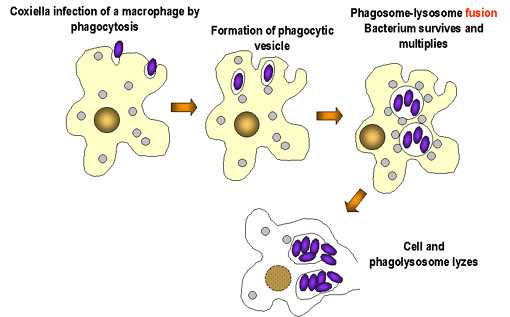 Coxiella are Gram-negative. Cell walls are composed of peptidoglycan. There are two cell types of this genus: large cell variants (LCVs) and small cell variants (SCVs). SCVs are rod-shaped and compact. A distinct features of the LCV is a spore-like particle (SLP), which is an electron-dense, membrane-bound polar body. Coxiella is an acidophilic bacteria, requiring a pH of 4.5-5 to grow. Morpholgically, Coxiella resembles Rickettsia. When it was first disovered, Coxiella burnetii was thought to be a member of this species, and named Rickettsia burnetii. Coxiella bacteria are obligate organisms, meaning they rely on their hosts for nutritional and environmental support. Sustained viability outside of the host organisms is unlikely. Coxiella reproduce by binary fission. They are durable organisms, capable of reproduction in the presence of chemicals such as acid hydrolases and defensins, which are bactericidal. It has been suggested that SLPs are released from degenerating LCVs, and that the SLPs are precursors to SCV cells. Ecology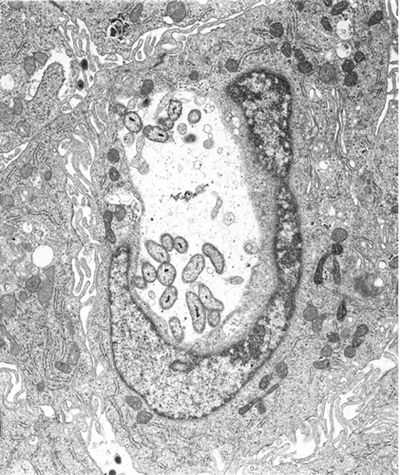 Coxiella burnetii, the most famous species of this genus, causes human Q fever (short for query fever). This disease was first described in 1935 by Edward Derrick, an Australian physician. It is carried by arthropods (particularly ticks), fish, birds, and mammals. C. burnetii appears to only cause disease in humans and laboratory animals such as rats and guinea pigs. However, it can induce abortions in sheep and goats, and causes reproductive problems in cattle. Humans typically contract Q fever by inhaling aerosoles contaminated with C. burnetii. Another source of human infection comes from drinking the milk of infected dairy cows. However, this is not as great of a risk if milk has been pasteurized. Q fever is not always a serious illness in immunocompetent individuals, and in some cases it is asymptomatic. Symptoms of Q fever resemble those of the flu. It can also lead to a mild form of pneumonia if the bacteria was contracted through inhalation. Tetracyclines are the preferred form of treatment for this disease. Q fever is a self-limiting disease that usually resolves itself within two weeks. In some cases, the disease can become chronic; this occurs in about 5% of victims. Chronic infection usually occurs in the heart; 60-70% of chronic infections are found in this organ. Endocarditis is a very common manifestation in chronic cases; there is a mortality rate of 50% associated with this illness. At one point it was suggested that Q fever left patients with an increase risk of ischaemic heart disease. However, the research of McCaughey et. al. (2005) shows otherwise. Another hypothesis was that a Q fever infection left patients with symptoms of chronic fatigue for up to ten years after infection. Studies of chronic fatigue resulting from Q fever remain inconclusive (Thomas et. al., 2004). While Q fever can occur in anybody, immunocompromised individuals are at an elevated risk for infection. C. burnetii is found all over the world, the exceptions being Antarctica and New Zeland. Although there are high amounts of C. burnetii antibodies in the immune systems of Japanese people, few cases of Q fever have been reported in Japan. It has been hypothesized that Japanese strains of C. burnetii have a low pathogenicity, although this has not been proven. Because only a small dose of the organisms is necessary for infection, C. burnetii has the potential to be a bioterrorism agent. ReferencesAndoh, Masako, Hiromi Nagaoka, Tsuyoshi Yamaguchi, Hideto Fukushi, and Katsuya Hirai. "Comparison of Japanese Isolates of Coxiella burnetii by PCR-RFLP and Sequence Analysis." Microbiol Immunol. 2004;48(12):971-5. Heinzen, Robert A. and James E. Samuel. "The Genus Coxiella." The Prokaryotes. Release 3.6. Accessed 29 July 2005. Mayer, Gene. "Bacteriology - Chapter Twenty-One: Rickettsia, Ehrlichia, Coxiella, and Bartonella." Microbiology and Immunology On-line: University of North Carolina School of Medicine. 11 January 2005. Accessed 1 August 2005. McCaughey, Conall et. al. "Lack of association between serological evidence of past Coxiella burnetii infection and incident ischaemic heart disease: nested case-control study." BMC Infectious Diseases 2005, 5:61. NIAID Biodefense Research. National Institute of Allergy and Infectious Diseases. Thomas, Hollie V., Daniel R. Thomas, Roland L. Salmon, Glyn Lewis, and Andy P. Smith. "Toxoplasma and coxiella infection and psychiatric morbidity: A retrospective cohort analysis." BMC Psychiatry. 2004; 4: 32. microbewiki.kenyon.edu Coxiella burnetii - wikidoc
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] OverviewCoxiella burnetii is an obligate intracellular bacterial pathogen, and is the causative agent of Q fever. The genus Coxiella is morphologically similar to Rickettsia, but with a variety of genetic and physiological differences. C. burnetii is a small Gram-negative bacterium that is highly resistant to environmental stresses such as high temperature, osmotic pressure, and ultraviolet light. These characteristics are attributed to a small cell variant form of the organism that is part of a biphasic developmental cycle, including a more metabolically and replicatively active large cell variant form.[1] It can survive standard disinfectants, and is resistant to many other environmental changes like those presented in the phagolysosome.[2] Pathogenesis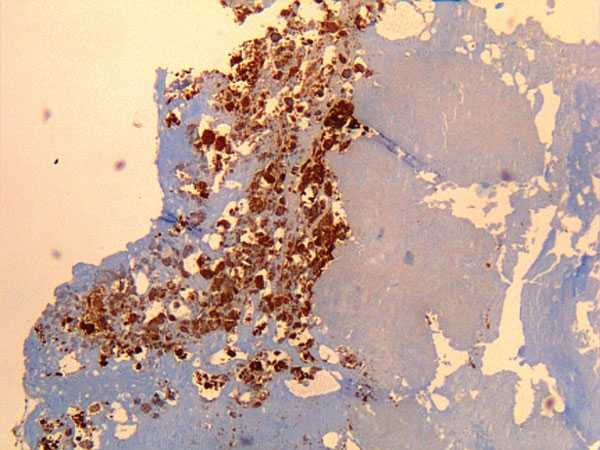 Immunohistochemical detection of Coxiella burnetii in resected cardiac valve of a 60-year-old man with Q fever endocarditis, Cayenne, French Guiana. Monoclonal antibody against C. burnetii and hematoxylin were used for staining. Original magnification ×50. Immunohistochemical detection of Coxiella burnetii in resected cardiac valve of a 60-year-old man with Q fever endocarditis, Cayenne, French Guiana. Monoclonal antibody against C. burnetii and hematoxylin were used for staining. Original magnification ×50. The ID50 (the dose needed to infect 50% of experimental subjects) is one via inhalation; i.e., inhalation of one organism will yield disease in 50% of the population. This is an extremely low infectious dose (only 1-10 organisms required), making C. burnetii one of the most infectious known organisms.[3][4] Disease occurs in two stages: an acute stage that presents with headaches, chills, and respiratory symptoms, and an insidious chronic stage. While most infections clear up spontaneously, treatment with tetracycline or doxycycline appears to reduce the symptomatic duration and reduce the likelihood of chronic infection. A combination of erythromycin and rifampin is highly effective in curing the disease, and vaccination with Q-VAX vaccine (CSL) is effective for prevention of it. [Source?] The bacteria use a Type IVB secretion system known as Icm/Dot to inject effector proteins called Ank proteins into the host. These effectors increase the bacteria's ability to survive inside the host cell. In Legionella pneumophila, which uses the same secretion system and also injects Ank proteins, survival is enhanced because these Ank proteins interfere with fusion of the bacteria-containing vacuole with the host's degradation endosomes.[5] GenomicsThere are currently at least 5 completely sequenced genomes of Coxiella burnetti [6] which contain about 2.1 Mbp of DNA each and encode around 2,100 open reading frames. 746 (or about 35%) of these genes have no known function. References
www.wikidoc.org Coxiella burnetii infection - Symptoms, diagnosis and treatmentNotifiable condition in the US and some other countries. People whose occupations put them at high risk of infection include abattoir workers, meat handlers, farmers, veterinarians, laboratory personnel, and military personnel. Symptoms and complications correspond to either an acute infection or persistent focalized infections. Infection during pregnancy may be associated with severe obstetric and fetal complications and endocarditis in the mother. Acute infection can be treated with a short course of doxycycline, but persistent focalized infections require long-term therapy with doxycycline plus hydroxychloroquine. Surgical resection of infected vascular tissue or prosthetic material may also be required. A zoonotic disease caused by the gram-negative, obligate, intracellular bacterium Coxiella burnetii . [1] Marrie TJ, Raoult D. Coxiella burnetii. In Mandell GL, Bennett JE, Dolin R, eds. Principles and practice of infectious diseases. 6th ed. Philadelphia, PA: Churchill Livingstone; 2005. [2] Hartzell JD, Wood-Morris RN, Martinez LJ, et al. Q fever: epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008 May;83(5):574-9. http://www.mayoclinicproceedings.org/article/S0025-6196(11)60733-7/fulltext http://www.ncbi.nlm.nih.gov/pubmed/18452690?tool=bestpractice.com [3] Eldin C, Mélenotte C, Mediannikov O, et al. From Q fever to Coxiella burnetii infection: a paradigm change. Clin Microbiol Rev. 2017 Jan;30(1):115-90. http://cmr.asm.org/content/30/1/115.long http://www.ncbi.nlm.nih.gov/pubmed/27856520?tool=bestpractice.com Many species of mammals, birds, and ticks are reservoirs of the bacterium, and the disease is spread globally through close contact with wild or domestic animals, especially their products of parturition, and also their urine, feces, or milk. However, C burnetii small cell variant (pseudospores) can spread by air up to 10 kilometers from the source of infection so that exposure history is frequently lacking. Symptoms and complications are different between acute infection (i.e., a self-limited febrile illness with varying degrees of pneumonia and hepatitis) and persistent focalized infections (e.g., endocarditis, vascular infection, osteoarticular infection, lymphadenitis). Infection during pregnancy has a specific clinical presentation (mostly asymptomatic), and may result in obstetric and fetal complications. [4] Million M, Roblot F, Carles D, et al. Reevaluation of the risk of fetal death and malformation after Q fever. Clin Infect Dis. 2014 Jul 15;59(2):256-60. https://academic.oup.com/cid/article/59/2/256/2895572 http://www.ncbi.nlm.nih.gov/pubmed/24748522?tool=bestpractice.com Commonly known as Q fever. bestpractice.bmj.com Coxiella Burnetii |authorSTREAMCoxiella burnetii Dr Neriman AYDIN Mikrobiyoloji ve Klinik Mikrobiyoloji Anabilim Dalı :Coxiella burnetii Dr Neriman AYDIN Mikrobiyoloji ve Klinik Mikrobiyoloji Anabilim Dalı*Rickettsiaceae ailesindendir *Gram negatif, zorunlu hücre içi bakteri *Pleomorfik yapıdadır *Kokoid yapıda veya basil şeklinde görülebilir * Spor benzeri form *Hareketsizdir, kirpikleri yoktur *Çeşitli enzimleri vardır *Antibiyotiklere duyarlıdır *Eklem bacaklılarda (artropod) hayatlarının bir bölümünü geçirirler:*Rickettsiaceae ailesindendir *Gram negatif, zorunlu hücre içi bakteri *Pleomorfik yapıdadır *Kokoid yapıda veya basil şeklinde görülebilir * Spor benzeri form *Hareketsizdir, kirpikleri yoktur *Çeşitli enzimleri vardır *Antibiyotiklere duyarlıdır *Eklem bacaklılarda (artropod) hayatlarının bir bölümünü geçirirlerCoxiella burnetii *1933 yılında Derrick, Avusturalyada izole etmiştir *Cox ve Burnet bakterinin riketsiyalar ile ilişkisini kanıtlamışlar *İkinci Dünya Savaşında salgınlar yapmış * Çok infektif Tek bakteri ile bulaş olabilmekte:Coxiella burnetii *1933 yılında Derrick, Avusturalyada izole etmiştir *Cox ve Burnet bakterinin riketsiyalar ile ilişkisini kanıtlamışlar *İkinci Dünya Savaşında salgınlar yapmış * Çok infektif Tek bakteri ile bulaş olabilmekteMorfoloji ve boyanma özellikleri *0,1-0,3 m boyutlarında, pleomorfik, hareketsiz, kapsülsüz, Gram negatif bakteri *Giemsa, Gimenez boyaları ile boyanır *Hücre duvarında içerisinde yoğun bir tabaka ve peptidoglikan içeren iki membrandan oluşur *Protein sentezi yapabilmektedir :Morfoloji ve boyanma özellikleri *0,1-0,3 m boyutlarında, pleomorfik, hareketsiz, kapsülsüz, Gram negatif bakteri *Giemsa, Gimenez boyaları ile boyanır *Hücre duvarında içerisinde yoğun bir tabaka ve peptidoglikan içeren iki membrandan oluşur *Protein sentezi yapabilmektedirPowerPoint Presentation:*Elektron mikroskobik olarak LCV (large cell variants) ve SCV (small cell varianats) *Her iki partikülde infeksiyözKültür özellikleri *10-12 günlük döletli yumurtanın sarı kesesinin membranında ürer *Çeşitli hücre kültürlerinde ürer *Hücreye fagositozla girerek fagolizozomlarda ürer :Kültür özellikleri *10-12 günlük döletli yumurtanın sarı kesesinin membranında ürer *Çeşitli hücre kültürlerinde ürer *Hücreye fagositozla girerek fagolizozomlarda ürerDirençlilik *UV, kuruluk ve ışınlara dayanıklıdır *%0,5 formaldehit ve %0,4’lük fenolde birkaç gün canlı kalabilir *60 °C’de 60 dakika canlı kalabilir *Su ve süt ürünlerinde, kuru kene dışkısında uzun süre yaşarlar * Soğukta saklanmış taze sütte bir aydan fazla calı kalır:Dirençlilik *UV, kuruluk ve ışınlara dayanıklıdır *%0,5 formaldehit ve %0,4’lük fenolde birkaç gün canlı kalabilir *60 °C’de 60 dakika canlı kalabilir *Su ve süt ürünlerinde, kuru kene dışkısında uzun süre yaşarlar * Soğukta saklanmış taze sütte bir aydan fazla calı kalırAntijenik yapı *Faz varyasyonu denilen antijenik değişiklikler yapar *Faz 1: Doğada ve deney hayvanlarındaki şeklidir, embriyonlu yumurta pasajları ile faz 2’ye geçer *Deney hayvanlarına faz 1 verildiğinde hem kendine hemde faz 2’ye antikor gelişir *Faz 2 verildiğinde kendine karşı antikor gelişir ve faz 1’e döner:Antijenik yapı *Faz varyasyonu denilen antijenik değişiklikler yapar *Faz 1: Doğada ve deney hayvanlarındaki şeklidir, embriyonlu yumurta pasajları ile faz 2’ye geçer *Deney hayvanlarına faz 1 verildiğinde hem kendine hemde faz 2’ye antikor gelişir *Faz 2 verildiğinde kendine karşı antikor gelişir ve faz 1’e dönerPowerPoint Presentation:Q humması tanısında IFA testinin yorumu Faz II antikor titresi Faz I antikor titresi YORUM IgG IgM IgG <1/100 Aktif Q hummas ı beklenmez >1/200 >1/50 0 Akut Q hummas ı (%100 prediktif) >1/800 Kronik Q hummas ı ( %98 prediktif) >1/1600 Kronik Q hummas ı (%100 prediktif)Virulansını etkileyecek plazmidlere sahiptir *QpRS plazmidi olanlar *Qph2 plazmidi olanlar *Plazmidi olmayanlar diye 3 gruba ayrılır *Akut infeksiyonlarda kloramfenikol ve tetrasikline duyarlı olanlar görülürken *Kronik infeksiyonlarda dirençlidir :Virulansını etkileyecek plazmidlere sahiptir *QpRS plazmidi olanlar *Qph2 plazmidi olanlar *Plazmidi olmayanlar diye 3 gruba ayrılır *Akut infeksiyonlarda kloramfenikol ve tetrasikline duyarlı olanlar görülürken *Kronik infeksiyonlarda dirençlidirYaptığı hastalıklar *Q ateşi, Q humması, Ouery ateşi *Pnömoni, *Uzayan ateş *Endokardit *Hepatit:Yaptığı hastalıklar *Q ateşi, Q humması, Ouery ateşi *Pnömoni, *Uzayan ateş *Endokardit *HepatitQ humması:Q humması Çoğunlukla asemptomatik Akut hastalık grip benzeri tablodan yoğun bakım gerektirecek ciddi pnömoni,hepatit Kronik infeksiyon esas olarak endokardit şeklinde, vasküler hastalıklar ve granülomatöz hepatit %2 hastada hastaneye yatış gerektiren tablolar Gebelik, immonusupresyon, kalp kapağı lezyonları ve vasküler anomaliler kronik Q hummasına zemin hazırlayabilmektedir Fransa’da en sık granülomatöz hepatit görülmekte ve ölümcül seyredebilmektedirLaboratuvar tanısı *Kesin tanı etken izolasyonu iledir 3. düzey biyogüvenlik olan laboratuvarlarda *Klinik örnek, embriyonlu yumurtaya veya kobaya intraperitoneal ekilir :Laboratuvar tanısı *Kesin tanı etken izolasyonu iledir 3. düzey biyogüvenlik olan laboratuvarlarda *Klinik örnek, embriyonlu yumurtaya veya kobaya intraperitoneal ekilirSerolojik tanı:Serolojik tanı KBD, ELİSA, İFA *1. haftadan itibaren antikor yükselir *Akut dönemde faz 2, kronik dönemde faz 1’e karşı antikorlar oluşur ELİSA ile tarama, İFA ile doğrulama testleri *Mikroaglütinasyon test de yapılırTedavi *Tetrasiklin *Kloramfenikol *Ko-trimoksazol:Tedavi *Tetrasiklin *Kloramfenikol *Ko-trimoksazolEpidemiyoloji *Tüm dünyada yaygındır *Meslek hastalığıdır *İnsana geçiş -toz partilülleri ile solunum yolundan, -çiğ süt içierek sindirim yolundan -deri ve mukozalardan *Hasta insanların solunum sekresyonları ve laboratuvar kazası ile insandan insana geçiş gösterilmiştir :Epidemiyoloji *Tüm dünyada yaygındır *Meslek hastalığıdır *İnsana geçiş -toz partilülleri ile solunum yolundan, -çiğ süt içierek sindirim yolundan -deri ve mukozalardan *Hasta insanların solunum sekresyonları ve laboratuvar kazası ile insandan insana geçiş gösterilmiştirPowerPoint Presentation:Coxiella burnetii IgM ve IgG sonuçları. Coxiella burnetii IgM Coxiella burnetii IgG Çalışma grubu Pozitif Negatif Toplam Pozitif Negatif Toplam Veteriner 2 (%2.2) 28 (%30.4) 30 (%32.6) 14 (%15.2) 16 (%17.4) 30 (%32.6) Celep 3 (%3.3) 29 (%31.5/ 32 (%34.8) 14 (%15.2) 18 (%19.6) 32 (%34.8) Kasap 2 (%2.2) 28 (%30.4) 30 (%32.6) 11 (%12.0) 19 (%20.7) 30 (%32.6) TOPLAM 7 (%7.6) 85 (%92.4) 92 (%100) 39 (%42.4) 53 (%57.6) 92 (%100) Türkiye’de sağlıklı bireylerde IFA ile yapılan çalışmalarda Faz II IgG araştırmalarında ise %7.1 - %9.2 pozitiflik saptanmıştırCoxiella burnetii’nin daoğada iki siklusu vardır:Coxiella burnetii’nin daoğada iki siklusu vardır*İxodes ve Argos cinsi keneler bakteriyi omurgalılara bulaştırır *Evcil hayvanlar infeksiyonu belirti vermeden geçirirler, idrar, dışkı ve sütleri ile yayarlar :*İxodes ve Argos cinsi keneler bakteriyi omurgalılara bulaştırır *Evcil hayvanlar infeksiyonu belirti vermeden geçirirler, idrar, dışkı ve sütleri ile yayarlarPowerPoint Presentation:İnfekte koyun plasentasının bir gramında >109 bakteri *Etkeni içeren toz partikülleri ile diğer memeliler ve insanlar infekte olurlarKorunma *İnsanlarda tam koruma sağlayan aşı geliştirilememiştir *Geliştirilen aşının insanlarda yan etkileri görülmüştür *Evcil hayvanların aşılanması ve sütlerin pastörizasyonu infeksiyon riskini azaltır:Korunma *İnsanlarda tam koruma sağlayan aşı geliştirilememiştir *Geliştirilen aşının insanlarda yan etkileri görülmüştür *Evcil hayvanların aşılanması ve sütlerin pastörizasyonu infeksiyon riskini azaltırwww.authorstream.com |
г.Самара, ул. Димитрова 131 [email protected] |
 |