|
|
||||||||||
|
Klebsiella pneumoniae. Чувствительность к антибиотикам klebsiella pneumoniaeKlebsiella pneumoniaeKlebsiella pneumoniae Gram Negatif, kendiliğinden hareketi olmayan, kapsül içerisinde, fermantasyon yapabilen bir bakteri türüdür.Normal flora olarak, ağız, deri ve bağırsakta olmasına rağmen, aspire edilmesi durumunda, akciğerde yıkıcı hasarlara neden olmaktadır.< Klinik olarak incelendiğinde Klebsiella sınıfının Enterobacteriaceae şubesinin bir üyesidir. Ayrıca K oxytoca ve K rhinoscleromatis bakterileride insanda enfeksyon yapan bakteri türleri arasında gösterilebilir. Son yıllarda K. Pneumoniae nazokomiyal enfeksyonlarda önemli bir patojen olmuştur. K. Pneumonia normal olarak toprakta bulunur ve soylarının 30%'u azot fiksasyonu ile aneorob olarak yaşamlarını sürdürürler. Diazotrof olarak serbest olarak yaşayan, K. Pneumoniae azot fiksasyonu için çok önemli olduğu, ve tarım ürünlerinin verimliğinde çok önemli rol üstlendiği görülmüştür. Klebsiella türünün üylereinde, hücre yüzeyinde iki tip antijen bulunur. İlk olanı, 9 çeşitte bulunan liposakkarit yapıdaki O antijenlerdir. İkincil olarak K antijeni 80 küsür çeşidi bulunan kapsüllü polisakkarit şeklinde olanlardır. Her iki serotip için de patojenik etkisi vardır. K. Pneumoniae, indole-negative testiyle ayrılan, ve her iki metazitos ve 3-hydroxybutyrate ile büyüme kabiliyeti olan K. Oxycota ile akrabalığı vardır. Konu başlıkları
TarihçesiDanimarkalı bilim adamı Hans Christian Gram (1853–1938), Gram boyama olarak bilinen tekniği geliştirmiş ve 1884'de K. Pneumoniea'dan Streptococcus pneumoniae'yı ayırt etmiştir. Klebsiella türü daha sonra Alman bakteriyolojist Edward Klebs (1834–1913) tarafından ismlenirilmiştir. Klinik ÖnemiK.pneumoniae, yoğun prülan balgam üreten, enflamasyon, hücre ölümleri ve hemoroji ile akciğerlerde kalıcı hasarlara sebep olabilir. Bu tipteki bakteriler orofaringeal mikroorganizma kolonilerinin alt solunum yolu extremitelerine aspire edilmesi ile kazanılır. Genel kural olarak, Klebsiella düşük immün sistemli kişilerde daha çok enfeksyona yol açar. Hastalık genellikle direnci zayıf orta yaşlı veya yaşlı kişileri etkiler. Hasta popülasyonu genellikle, respritüel konak savunması azalan, diyabet, alkolizm, kanser, karaciğer hastalığı, KOAH, glükokortikoid terapisi ve kronik böbrek yetmezliği olan hastalarda bulaşma oranı yüksektir. Bu bakteri hastanede yatan bir hastadan diğer hastaya geçebilmektedir. Feçes en önemli bulaşma yoludur ve kontamine eşyaların kullanımı da enfeksyon bulaşma riskini artırmaktadır. Hastane dışında Klebsiella bakterisinin neden olduğu en yaygın enfeksyon pnömoni'dir. En tipik şekli ise bronkopnömoni ve bronşittir. Bu hastalar, akciğer absesi, ampiyemi, kavitasyon ve üral adhezyon gelişmesine meylederler. Antimikrobiyal tedaviye karşın ölüm oranı %50'dir. Mortalite oranı en yüksek olanlar hastalar alkolizm ve bakteriyemi ile mücadele eden hastalardır. Pnömoniye ek olarak, Klebsiella Üriner sistemde, safra kanalında ve cerrahi girişim yapılan yerde enfeksyona sebep olabilir. Klinik hastalık aralığı olarak, tromboflebit, idrar yolu enfeksiyonu (İYE), kolesistit, ishal, üst solunum yolu enfeksiyonu, yara enfeksiyonu, osteomiyelit, menenjit, bakteriyemi ve septisemiyi içermektedir. İnvaziv bir cihaz veya komponent olan hastalrda, cihazın kontamine olma riski yüksektir; solunum destek ekipmanları ve üriner katater risk faktörü yüksek olan hastalar kategorisine koyar. Antibiyotik kullanımı da nazokomiyal olarak Klebsiella'nın etkinliğini artırır. Bakterinin kana bulaşması sepsis veya septik şok gelişir. Klebsiella'nın rinoskleroma ve kronik atrofik rinit adında iki tana olağandışı hastalığı mevcuttur. Rinoskleroma nazofarenks'in kronik enflamasyon sürecinde rol oynayan bir hastalıktır. Kronik atrofik rinit ise nazal mukoza'nın nekroze uğramasını ifade eder. Klebsiella yaşlı kişilerde idrar yolu enfeksyonu için E.Coli'den sonra ikinci sırada yer almaktadır. Ayrıca, kronik akciğer hastalığı, enterik patojenik, burun mukozası atrofisi ve rinoskleroma olan hastalar için bir fırsatçı patojendir. K. pneumoniae'nın eni antibiyotik karşı dirençli suşlar ortaya çıkmaktadır. Rezistant SuşlarıKlebsiella organizması çoğu antibiyotiğe karşı dirençlidir. Mevcut kanıtlar, dirençli genlerin kaynağı olarak, bir plazmitin etkisi olduğunu göstermektedir. Geniş spektrumlu beta-laktamaz GSBL üretme yeteneği olan Klebsiella çoğu antibiyotiğe direnç göstermektedir. Çoğunlukla direnç gösterdikleri aminoglikozitler, florokinolonlar, tetrasiklinler, Kloramfenikol ve Trimetoprim-sulfametoksazoldür. Karbapeneme dirençli Enterobacteriaceae (CRE) veya karbapenemaz üreten Enterobacteriaceae ile enflamasyon sağlık alanında önemli bir sorun olarak ortaya çıkmaktadır. Karbapenem dirençli Enterobacteriaceae'lerin birçoğu (CRE) bir karbapenem dirençli Klebsiella pneumoniae (CRKP) olduğundur. Geçen on yıl içersinde, dünya çapında CRKP'nin sürekli bir artış eğiliminde olduğu görülmüştür; Ancak bu yeni çıkan nazokomiyal enfeksiyon, 2006 yılında İsrail'de en iyi sağlık koşulları olarak bilinen bir dönemde salgın oluşturmuştur. İlk olarak ABD'de Kuzey Caroline'de tanımlanmıştır. O zamandan beri CRKP 41 eyalette tespit edilmiştir; New York ve New Jersey gibi bazı hastanelerde rutin olarak kazanılmıştır. Şimdi ise Amerika Birleşik Devletleri içerisinde en sık karşılaşılan CRE türdür. CRKP neredeyse tüm antimikrobiyal ajanlara dirençlidir ve CRKP ile enfekte olmuş hastaların uzun süreli yatşları, invaziv girilşimlere ve cihazlara (örneğin; ventlatörler, santral venöz kataterler) maruz kalan hastalarda morbidite ve mortalite oranının yüksek olmasına neden olmuştur. Karbapanem dirençli suşları ile mücadelede son çare olarak kullanılan ilaçlardandır. Kücük yeni mutasyonlar eğer enfeksyona sebep oluyorsa, sağlık çalışanılarının bu dirence karşı yapabilecekleri çok az şey vardır. Kaynakça
Klebsiella pneumoniae Hakkında BilgiKlebsiella pneumoniaeKlebsiella pneumoniaeKlebsiella pneumoniae Hakkında Video Klebsiella pneumoniae konusunu görüntülemektesiniz.Klebsiella pneumoniae nedir, Klebsiella pneumoniae kimdir, Klebsiella pneumoniae açıklaması There are excerpts from wikipedia on this article and video www.turkaramamotoru.com Klebsiella pneumoniae pathogenesis - microbewikiUniversity of Oklahoma Study Abroad Microbiology in Arezzo, Italy[1]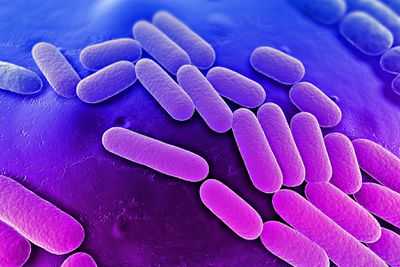 Scanning electron microscope image of Klebsiella pneumoniae. From: Bioquell.com [2] Scanning electron microscope image of Klebsiella pneumoniae. From: Bioquell.com [2] Etiology/BacteriologyTaxonomy| Domain = Bacteria | Phylum = Proteobacteria | Class = Gammaproteobacteria | Order = Enterobacteriales | Family = Enterobacteriaceae | Genus = Klebsiella | Species = K. pneumoniae [1] DescriptionKlebsiella pneumoniae is a gram-negative, non-motile, lactose fermenting, rod-shape organism. K. pneumoniae is able to grow either with or without free oxygen, deeming it a facultative anerobe. This organism is also surrounded by a capsule, which increases its virulence by acting as a physical barrier to evade the host’s immune response. This capsule also protects the cell from dessication. K. pneumoniae is a home-grown microorganism in that it is resides in the microbiota of humans. It can be found in the mouth, skin, and intestinal tract, where it initially does not cause disease. Although found in the microbiota, K. pneumoniae can progress into severe bacterial infections leading to pneumonia, bloodstream infections, wound infections, urinary tract infections, and meningitis. Patients who require equipment such as catheters or ventilators are at high risk for infections. Also, a patient administered a course of broad-spectrum antibiotic treatment is at an even high risk due to the disruption of the normal flora of the bacteria in the body, deeming it more susceptible to pathogens [1]. Klebsiella pneumoniae was named afer Edwin Klebs (1834-1913), a 19th century German microbiologist [2]. Although he was not the first to isolate K. pneumoniae, naming a genus after him honored his work with Corynebacterium diptheriae. Around the same time, Hans Christian Gram (1853-1938), created a microbiological technique known as the Gram stain in 1884 to differentiate between K. pneumoniae and S. pneumoniae [3]. PathogenesisTransmissionKlebsiella infections is spread through exposure to the bacteria via respiratory tract, which causes pneumonia, or the blood to cause an infection in the bloostream. Klebsiella infections are most well-known in hospitals spread through person-to-person contact by contaminated hands of surrounded people in the hospitals, whether it be an employee or a patient. Klebsiella is spread very easily and rapidly, but not through the air. Healthcare settings are most vulnerable to Klebsiella infections due to the nature of procedures that allow easy access of bacteria into the body. Patients who are on ventilators, catheters, or surgery wounds are highly prone to catching this deadly infection [4]. Incubation/Infectious Dose/ColonizationIn humans, the infectious dose is not known. As for the incubation period, it is also not fully understood but possibly arises within a number of days [4]. Klebsiella bacteria are found widely through nature in soil and water. In regards to human, K. pneumoniae is prevalent in normal microbiota of the intestinal tract and colon, but not in alarmingly high numbers. They may also be found in the mouth and the skin [3]. Infection of K. pneumoniae occur in the lungs, where they cause necrosis, inflammation, and hemorrhage within the lung tissue. This is caused by aspirating oropharyngeal microorganisms into the lower respiratory tract. Hospital-acquired infections rely on the urinary tract, lower respirator tract, biliary tract, and surgical wounds to set up colonization [5]. EpidemiologyWhen dealing with hospital-acquired bacterial infections caused by K. pneumoniae can arise in different parts of the body and in different forms of illness depending on transmission. K. pneumoniae is responsible for 6-17% of UTI’s, 7-14% of pneumonia, 4-15% of septicemia, 2-4% of wound infections, 4-17 nosocomial infections in intensive care units, and 3-20% of all neonatal septicemia cases. All of these cases rank within at least the top 11 in comparison to all other bacterial pathogens. In the United States, people who suffer from alcoholism make up 66% of people affected by community-acquired pneumonia. K. pneumoniae is now among the top 8 pathogens in hospitals and is a rising issue among hospitals all around the world due to antibiotc resistance. In humans, K. pneumoniae resides in the nasopharynx and in the intestinal tract. Since gram-negative bacteria do not have good growth on human skin, they are rarely found there in comparison to internal parts of the body. Reported carrier rates are quire the opposite of this fact, when in a hospital environment. Carriers rated in hospitalized patients were 19% in the pharynx, 77% in the stool, and 42% on the hands. Even hospital employees had elevated rates of carriage to K. pneumoniae. These findings were linked to the over usage of broad-spectrum antibiotics rather than delivery of care [6]. In 2011, an investivation of Klebsiella pneumoniae carbapenemase (KPC)-producing Enterobacteriaceae was conducted in hospitals among patient with short and long-term stays. Over a 1-year period, KPC-producing Enterobacteriaceae was found throughout 4 counties in Indiana and Illinois. The source of the problem was found to be within long-term facilities and patients [5]. KPC has been found in a total of 44 states thus far [7]. 14% of bacteremia cases are because by K. pneumoniae, which places it in second place next to Escherichia coli for origins of gram-negative sepsis. Outbreaks of neonatal septicemia and K. pneumoniae can be found worldwide. In Israel, a number of hospital facilities reported increases in KPC-producing Enterobacteriaceae beginning in 2006, while the first case in the United States was reported in 2001[8]. Virulence FactorsCapsular Polysaccharide and Lipopolysaccharide O Side ChainLipopolysaccharide (LPS) and capsular polysaccharide (CPS) are two of the most important virulence factor of K. pneumoniae in causing sepsis. To resist complement-mediated killing, LPS contains lipid A, core, and O-polysaccharide antigen. CPS is essentially the outer layer of the pathogen containing polymorphonuclear cells, which creates resistance against phagocytosis. CPS reduced interaction between bacterial cells by reducing the amount of C3 being put on the bacteria and by acting as a barrier to obstruct contact between macrophage receptors and their ligands on the bacterial surface. CPS is absolutely critical and also modulates interaction between surfactanct protein D (SP-D) and K. pneumoniae. SP-D mediates aggregation and improves phaocytic clearance by human alveolar macorphages. With CPS, C3 and SP-D cannot attach, which creates clearant of the microorganism from the lower respiratory tract, resulting in pneumonia 9. Capsular AntigensK. pneumoniae use capsular antigens composed of complex acidic polysacchardes, which are essential to the virulence to this pathogen. The capsular antigens can be classified into 77 serological types and are often made up of uronic acids. As a mechanism of protection, the bacterium evades phagocytosis by utilizing thick bundles of fibrillous structures by polymorphonuclear granulocytes. This mechanism also prevents killing by bactericidal serum factors and inhibits complement constituents, such as C3b, which opsonize the pathogen. These effective virulence factors also have the ability to inhibit the differentiation capacity of macrophages in vitro 9. Antibiotic Resistant Strains Geographic spread of K. pneumoniae isolates exhibiting resistance to third-generation cephalosporins (G3CRKP) and carbapenems (CRKP) between 1999 and 2010. From: cddeo.org [3] Geographic spread of K. pneumoniae isolates exhibiting resistance to third-generation cephalosporins (G3CRKP) and carbapenems (CRKP) between 1999 and 2010. From: cddeo.org [3] KPC Outbreak at NIHIn 2011, Klebsiella pnuemomiae Carbapenamase arrived in one of the most established and renowned hospitals, the Clinical Center at the National Institutes of Health in Bethesda, Maryland, known as the NIH. A 43-year-old woman was transferred to the NIH, who had lung transplant complications and a KPC infection. With no known, outbreak, NIH employee were relieved after clearing the infection and discharging the patient. Weeks later, a new patients tested positive for KPC, but with no know link to patient number one. Over the next six months, several patients were showing up positive for KPC and eventually quarantined from the rest of the hospital. Doctors began administering combination antibiotics, older formulas from decades back, and even experimental antibiotics. None of these methods seemed to work and ultimately, a total of 18 patients became infected with KPC. 6 patients died from KPC and the outbreak came to a halt just as fast as it started. Genetic analysis was used to understand the transmission sequence, The bacteria is thought to be home grown in that we have K. pneumoniae living in our digestive systems with a gene that can spread resistance to different bacteria. Today, the NIH knows that KPC is still lurking in the hospital. They only know that they must be extremely vigilant in the future due to the increasing rise of KPC infections in the United States 10]. Clinical FeaturesSymptomsKlebsiella infections are prominent in individuals who have diabetes or who suffer from alcoholisms due to a weakened immune system. K. pneumoniae is also a well-known cause of community-acquired pneumonia. Symptoms associated with community-acquired K. pneumoniae include sudden onset, high fever and a jelly-like sputum. Shortness of breath and coughing are also common symptoms. If introduced into the bloodstream, K. pneumoniae has the capability of causing meningitis, which affect the CNS. Symptoms include sharp head pain, nausea, dizziness, and impaired memory. Urinary tract infections alongside bacteremia are leading consequences of Klebsiella infections as well. An alarming issue and even more common infection originating from K. pneumoniae are nosocomial infections. Hospital patients who have extended stays in Intensive Care Units, who are either receiving long courses of broad spectrum antibiotics or have additional health issues are at risk for attaining a nocosomial infection. Broad spectrum antibiotics introduced into the microbiome, in turn, creates antibiotic resistance for K. pneumoniae to thrive and colonize. Many hospital patients and even faculty members who acquire K. pneumoniae infections will develop pneumonia, bacteremias, and urinary tract infections [3]. The emergence of CRE producing the Klebsiella pneumonia carbapenase (KPC) enzyme are not increasingly common all around the United States. Antibiotic resistance is now a serious problem in hospitals, withincreased mortality rates and decreased options of treating the infection. DiagnosisK. pneumoniae may be isolated from blood, urine, pleural fluid, and wounds. By simply gram staining a sputum sample obtained from a patient could lead one to diagnosing K. pneumoniae. Cultures should be obtained from sites such as open wounds, peripheral or central intravenous access sites, urinary catheters, and respiratory equipment. Chest radiography can also lead the way in diagnosing a K. pneumoniae infection. The organism usually resides in one of the upper lobes of the lungs, but may also be involved in lower portions as well. The lobe will appear swollen and can, in many cases, produce abscesses. TreatmentAntibiotic TherapyUnfortunately, K. pneumoniae is resistant to a number of antibiotics, deeming treatment options very limited. Choosing an antibiotic treatment for K. pneumoniae depends on the organ system that has been targeted. The choice is especially modified for people with confirmed bacteremia. Antibiotics with high intrinsic activity against K. pneumoniae include cephalosporin, carbapenems, aminoglycosides, and quinolones. These treatments are initially used as monotherapy or even as a combination. For patients who are severely ill, an initial course, usually between 48-72 hours of combination aminoglycoside therapy, is suggested. This should then be followed by an extended-spectrum cephalosporin 9.  Two plates are shown growing bacteria in the presence of discs containing various antibiotics. The left plate is susceptible to antibiotics and shows no growth. The plate on the right has a CRE that is resistant to all of the antibiotics tested. From: cdc.gov [4] Two plates are shown growing bacteria in the presence of discs containing various antibiotics. The left plate is susceptible to antibiotics and shows no growth. The plate on the right has a CRE that is resistant to all of the antibiotics tested. From: cdc.gov [4] ResistanceCarbapenem resistance is a emerging issue and is notably due to K. pneumoniae carbapenemase beta-lactamase (KPC). With the spread of KPC-producing bacteria, clinicians are dependent on polymyxins and tigecycline for treatment. Polymyxins have been the only agents active against KPC-producing bacteria. However, they were used infrequently due to their association with nephrotoxicity and neurotoxicity. Only a small amount of data support Polymyxin as a quality treatment for KPC. During a Manhattam outbreak caused by KPC-producing K. peumoniae, three bloodstream infections were treated with one survivor from the group. This drug is often used in combination with other antimicrobials 11. SurgerySurgey may be needed for patients who experience empyema, lung anscess, pulmonary gangrene, or respiratory tract obstruction following a Klebsiella infection. Correction of posterior urethral valves in patients with reoccurring UTIs is a possibility or other abnormalities influenced by infection 5. PreventionTo prevent the spread of infections, patients should remain very cautious of their handwashing habits. Handwashing with soap and water should happen in the following instances: before touching eyes, nose, or mouth, before preparing food, before addressing bandaged or wound areas, after using the restroom, and after using the restroom. It is especially important to be cautious when entering and exiting hospital rooms. Be sure to use proper handwashing techniques after touching doorknobs, bed rails, using hospital restroom, and interacting with sick patients, even if they are loved ones. Host Immune ResponseIL-12, STAT4, IFN-γ, and IL-17 signaling have each been shown to be critical for host defense against pulmonary K. pneumoniae infection12. References1. UniProt. Taxonomy: Species Klebsiella pneumoniae. Available at http://www.uniprot.org/taxonomy/990925. 2. Centers for Disease Control and Prevention. Klebsiella pneuminae in Healthcare Settings. Available at http://www.cdc.gov/HAI/organisms/klebsiella/klebsiella.htm.3. Department of Health and Hospitals. Available at http://new.dhh.louisiana.gov/assets/oph/Center-PHCH/Center-CH/infectious-epi/EpiManual/KlebsiellaManual.pdf. 4. Boston Medical Research Occupational Health Program. Available at http://www.bu.edu/rohp/files/2012/08/KPC-Klebsiella.pdf. 5. Medscape. Klebsiella Infections. Available at http://emedicine.medscape.com/article/219907-overview#a01995. 6. Klebsiella spp. As Nosocomial Pathogens: Epidemiology, Taxonomy, Typing Methods, and Pathogenicity Factors Clin Microbial Rev. Oct 1998; 11(4): 589-603.7. A Superbug Outbreak At NIH. Available at http://www.pbs.org/wgbh/pages/frontline/health-science-technology/hunting-the-nightmare-bacteria/a-superbug-outbreak-at-nih/. 8. Carbapenem-Resistant Enterobacteriaceae: Epidemiology and Prevention. Oxford Jounals. Clinical Infectious Diseases. Mar 2011; 53(1): 60-67. 9. American Society for Microbiology: Infection and Immunity. Molecular Analysis of the Contribution of the Capsular Polysaccharide and the Lipopolysaccharide O Side Chain to the Virulence of Klebsiella pneumoniae in a Murine Model of Pneumonia. Available at http://iai.asm.org/content/70/5/2583.full?maxtoshow=&HITS=10&hits=10&RESULTFORMAT=&titleabstract=Molecular+analysis+of+the+contribution+of+the+capsular+polysaccharide+and+the+li&searchid=1&FIRSTINDEX=0&resourcetype=HWCIT. 10. Hunting the Nightmare Bacteria. Available at http://www.pbs.org/wgbh/pages/frontline/hunting-the-nightmare-bacteria/. 11. Emergence of Klebsiella pneumoniae Carbapenemase (KPC)- Producing Bacteria- South Med J. Jan 2011; 104 (1): 40-45. 12. Divergent Roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae J Exp Med. Sep 2005. 202(6): 761-769. Created by Victoria Ann Kappel Student of Dr. Tyrrell Conway, University of Oklahoma microbewiki.kenyon.edu Klebsiella pneumoniae » Gram-Negative Bacteria » Pathogen Profile Dictionary | Klebsiella pneumoniae is a Gram-negative, non-motile, facultative anaerobe belonging to the Enterobacteriaceae family of the γ-Proteobacteria class in the phylum Proteobacteria. K. pneumoniae consists of straight rods 1 to 2 μm (micrometres) in length with a thick, surrounding capsule (Figure 1). When cultured, this species produces a distinctive yeasty odor and bacterial colonies have a viscous/mucoid appearance (Figure 2). K. pneumoniae is commonly found in the human digestive tract as part of the natural microflora, and is often the cause of hospital acquired, or nosocomial infections involving the urinary and pulmonary systems, especially since it is able to adapt to an existence in an oxygenated or deoxygenated environment. Immunocomprimised individuals (people with AIDS or cancer) infected with K. pneumoniae usually develop respiratory tract infections such as pneumonia, but blood infections (septecemia), wound or surgical site infections, and meningitis are also possible.
Figure 1. This scanning electron micrograph reveals some of the ultrastructural morphologic features of a Klebsiella pneumoniae bacterium.
Figure 2. After 24 hours, this inoculated MacConkey agar culture plate cultivated colonial growth of Gram-negative, small rod-shaped and facultatively anaerobic Klebsiella pneumoniae bacteria. This pathogen possesses many virulence factors that allow it to go undetected by the host's immune system and cause infection in a variety of ways. Firstly, this species uses ferric-siderophore receptors of the host to activate their enterobactin-mediated iron-sequestering system, allowing for bacterial growth. Their thick polysaccharide capsule prevents ingestion by phagocytes and their somatic antigens from being detected by the host’s antibodies. Also, serum complement activation is more difficult with the thick lipopolysaccharide capsule it possesses (Greenwood et al., 2002). In fact, K. pneumoniae avoids damage by complement proteins by the extreme length of the molecules comprising the capsule, essentially allowing the lytic C5b-9 (complement) complex to form too far away from the membrane. This prevents opsonization and membrane attack complex (MAC) insertion, which leads to lysis of the bacterium (Figure 3).
Figure 3. A schematic depicting a capsule from allowing C3b complement protein from initiating the complement cascade. There are many metabolic features of K. pneumoniae that make it unique. To begin, this bacterium produces an enzyme called carbapenemase, which makes it resistant to the drug carbapenem. This species also produces bacteriocins, which are proteinaceous toxins produced by bacteria to inhibit the growth of similar or closely related bacterial strains (Greenwood et al., 2002). Since K. pneumoniae is normally found in the microflora of the host, the production of bacteriocins could actually be harmful, as it may get rid of the essential or 'good' bacteria found in the intestines. K. pneumoniae also can utilize sodium citrate and can decarboxylate some amino acids to form amines (Forbes et al., 2002). Two other metabolic featuresinclude the pathogen's ability to hydrolyze the glycoside esculin and its inability to split tryptophan to form indole (Greenwood et al., 2002). The morphology, culture morphology, motility, and metabolic activities can all be used to identify K. pneumoniae. Once the bacteria are mounted and stained, straight rods 1 to 2 μm in length should be visible under a light microscope (Figure 4). Once the species is cultured, there should be a distinct yeasty odor, as well as a viscous/mucoid appearance (Figure 2). To ensure the absence of motile structures such as flagella, a motility test can be performed, involving inoculation and incubation a semi-solid agar deep test tube (Forbes et al., 2002). Test results should show that the bacteria remain at the site of inoculation. Also, a flagella stain can be performed, which should show the absence of the motile structure. When Simmons citrate agar is inoculated and incubated, growth should occur, changing the bromthymoll blue indicator from green to blue, indicating the metabolic feature of citrate utilization (Forbes et al., 2002).
Figure 4. Photomicrograph of Klebsiella pneumoniae bacteria depicting rod-shape morphology. Another metabolic feature of K. pneumoniae is its ability to decarboxylize amino acids; this can be tested by inoculating decarboxylase broths with arginine, lysine, and ornithine. After incubation, the results should show a colour change from yellowish-orange to purple, indicating an alkaline pH (>8.0) due to the decarboxylation of the amino acids. As mentioned earlier, K. pneumoniae is able to hydrolyze esculin, which can be tested by inoculating and incubating a medium with this bacterium. This medium should become blackened, and when examined under a Wood’s lamp, there should be a loss of fluorescence (Forbes et al., 2002). This bacteria's morphology, cultural morphology, motility, and metabolic features allow for its identification and possible treatment.
References: Forbes, B.A., Sahm, D.F., & Weissfeld, A.S. (2002). Bailey and Scott’s diagnostic microbiology (11th ed.). St. Louis, MO: Mosby. Greenwood, D., Slack, R.C.B., & Peutherer, J.F. (2002). Medical microbiology: A guide to microbial infections: Pathogeneisis, immunity, laboratory diagnosis and control (16th ed.). Toronto, ON: Churchill Livingstone. |
г.Самара, ул. Димитрова 131 [email protected] |
 |